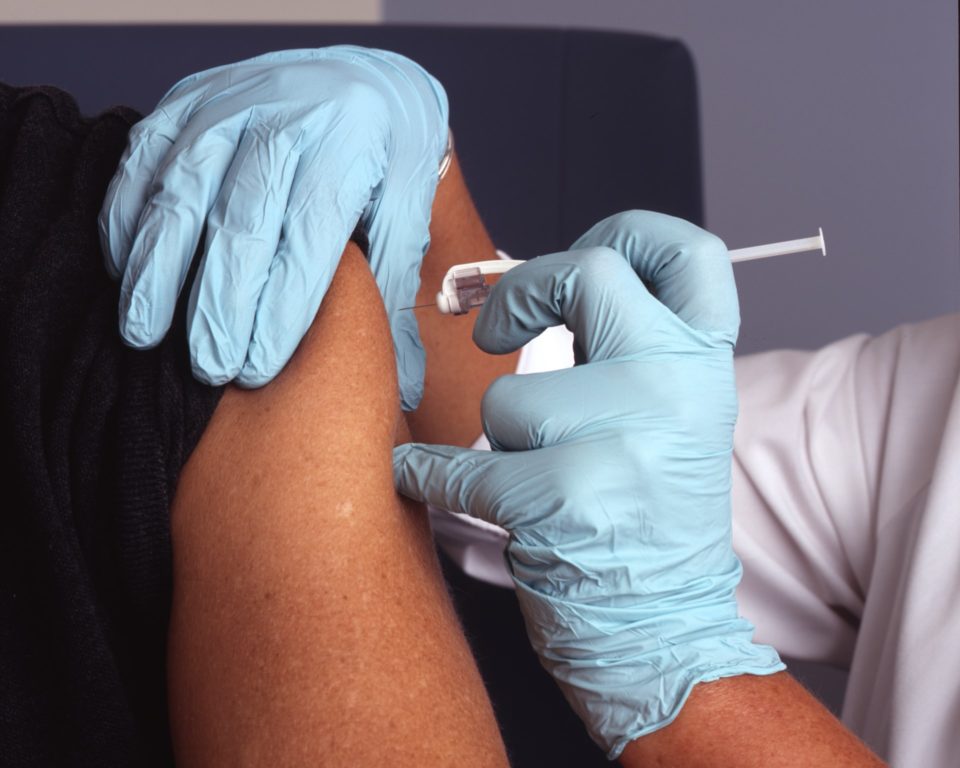
A global study on an injectable HIV pre-exposure prophylaxis (PrEP) drug has been stopped early after researchers found the drug to be highly effective.
A long-acting antiretroviral drug administered as an injection every two months has been found to offer ‘powerful’ protection in uninfected people from HIV in a large-scale study.
The apparent success is said to offer a potentially easier alternative to taking daily pills of other antiretrovirals, which has proved difficult for many people.
The drug known as Cabotegravir (CAB) is manufactured by ViiV Healthcare, a pharmaceutical company created as a joint venture by Pfizer and GlaxoSmithKline to specialise in the development of therapies for HIV infection.
If a treatment or prevention trial is stopped early, it means the news is either very good or very bad.
It means the new intervention is either harmful, pointless – or so good, it would be unethical not to offer it.
For instance, early this year (February) one of the largest HIV vaccine trials was stopped after the HVTN 702 (Uhambo) trials on an experimental vaccine showed that the vaccine was no better than the placebo, meaning that there had been no difference in the HIV infection rate between people receiving the vaccine and people receiving a placebo.
In the case of CAB the scientists have hailed the early termination of the HPTN 083 study as good news which may fundamentally change the nature of HIV PrEP, as well as HIV prevention. In the study, a large and carefully-designed trial, injections every two months of cabotegravir stopped seven out 10 (69 per cent) more HIV infections than what has been up till now, state-of-the-art PrEP, namely a daily dose of the pill formerly known as Truvada.
The strategy of uninfected people taking drugs to ward off HIV, known as pre-exposure prophylaxis (PrEP), earned Kenya’s Ministry of Health’s blessing in 2016 when it included oral PrEP in its antiretroviral therapy (ART) guidelines for people at substantial risk of HIV infection.
In 2017 the Health Ministry published a PrEP implementation framework and has been rolling out PrEP across the public health sector since.
PrEP was rolled out to roll out a new drug meant to protect HIV-negative people at high risk of contracting the virus before being made accessible to the rest of the population.
“It’s really exciting,” Jared Baeten, an epidemiologist at the University of Washington, Seattle, who was not involved in the NIAID trial but conducted a landmark PrEP study of anti-HIV pills (one was Truvada) in Kenya and Uganda told Science Magazine.
“It gives another option for people who can’t or don’t want to take daily pills.”
In the new study named HPTN083, Truvada was tested against intramuscular injections of the experimental drug and placebos in men who have sex with men and transgender women.
The trial, sponsored by the US National Institute of Allergy and Infectious Diseases (NIAID), began in December 2016 and enrolled more than 4500 participants worldwide, who were randomly assigned to Truvada, cabotegravir, placebo pills, or placebo injections.
On a statistical level, the study which is yet to be published in a peer-reviewed journal or presented to HIV researchers at a meeting did not demonstrate cabotegravir’s superiority over Truvada, but the data clearly showed it worked just as well.
Having options may get more people to try PrEP, Baeten said, comparing the situation to women who now have several forms of contraception from which to choose.
“IAVI commends all the dedicated trial participants, sponsors, clinicians, staff, researchers, and scientists on encouraging results from HPTN 083 & looks forward to data from HPTN 084,” said the International AIDS Vaccine Initiative (IAVI) in a Tweet.
Kenya is among seven African countries that were picked to carry out large-scale trials similar to the HPTN 083. The HPTN 084 trials, however, are mainly focused on showing whether the cabotegravir (CAB) jab can prevent HIV infection among uninfected women.
Besides Kenya, the clinical trials were launched in 2017 among 3,200 HIV-uninfected, sexually active women in six other countries — Uganda, Botswana, Malawi, Swaziland, South Africa, and Zimbabwe.
The HPTN 084 which is expected to end next year is the first large-scale clinical trial of an injectable medication for HIV prevention in sexually active women aged 18 to 45 years meant to examine the safety and effectiveness of the injectable drug compared to the daily oral PrEP pills. The four and a half years study is still ongoing.
There's no story that cannot be told. We cover the stories that others don't want to be told, we bring you all the news you need. If you have tips, exposes or any story you need to be told bluntly and all queries write to us [email protected] also find us on Telegram